COVID-19 News and Views from a socially distanced scientist
Published:
As of March 19, 2020 it’s been about a week since widespread closures began in my area—Ann Arbor, Michigan. It’s been 8 days since the World Health Organization declared a pandemic. I’m continuing to curate the top (in my mind) science news and resources here on the blog. You can see my first few days of updates here! I’m trying to add explainers for non-scientists and adding my take on the significance of findings when possible. As with anything on the internet, readers should take my interpretations and editorializations with an ounce of skepticism. What started as advocating for a few weeks of social distancing, now looks more like an effort to present relevant science as humanity battles a potentially long-lasting pandemic. I hope it helps in some way.
March 21, 2020 Update
First, an important call to action. Personal pleas from health care workers regarding the lack of personal protective equipment (PPE) have me very worried. As do articles by the NY Times and Forbes. While there are contingency strategies from the Food and Drug Administration (FDA), this will not keep our healthcare workers adequately safe. Harvard estimates indicate our hospitals will likely be at capacity or above capacity for months to come—see the figure below. It is unconscionable to not take care of the health professionals at the front lines of critical care. There is a petition you can sign. The Coalition to Protect America’s healthcare has an easy way to send messages. In addition, the American College of Emergency Physicians has a website allowing physicians to easily send an email to elected officials. Based on that, I’ve provided an example script you can adapt for email or use to call your representatives.
Hello, I’m FIRST,LAST and a constituent from TOWN/ZIP. I am reaching out to ask REPRESENTATIVE NAME to provide urgent help for health care workers on the front lines of caring for COVID-19 patients. Emergency physicians and health systems currently face severe shortages of personal protective equipment like N95 masks that will leave many health professionals insufficiently protected in the midst of this outbreak. We need to take action now to fill this gap with every resource we have available, including from the Strategic National Stockpile (SNS). Although the Administration announced that PPE production is ramping up, doctors need these supplies now, and will need significantly more as cases multiply. In order to ensure that emergency physicians and other frontline healthcare providers remain healthy and able to continue to provide vital patient care, the Administration and Congress must exhaust every option available to increase PPE production and prioritize distribution to emergency physicians and other frontline providers. I ask REPESENTATIVE NAME to urge their colleagues and the Administration to immediately address the PPE shortage. Thank you for your hard work answering the phones.
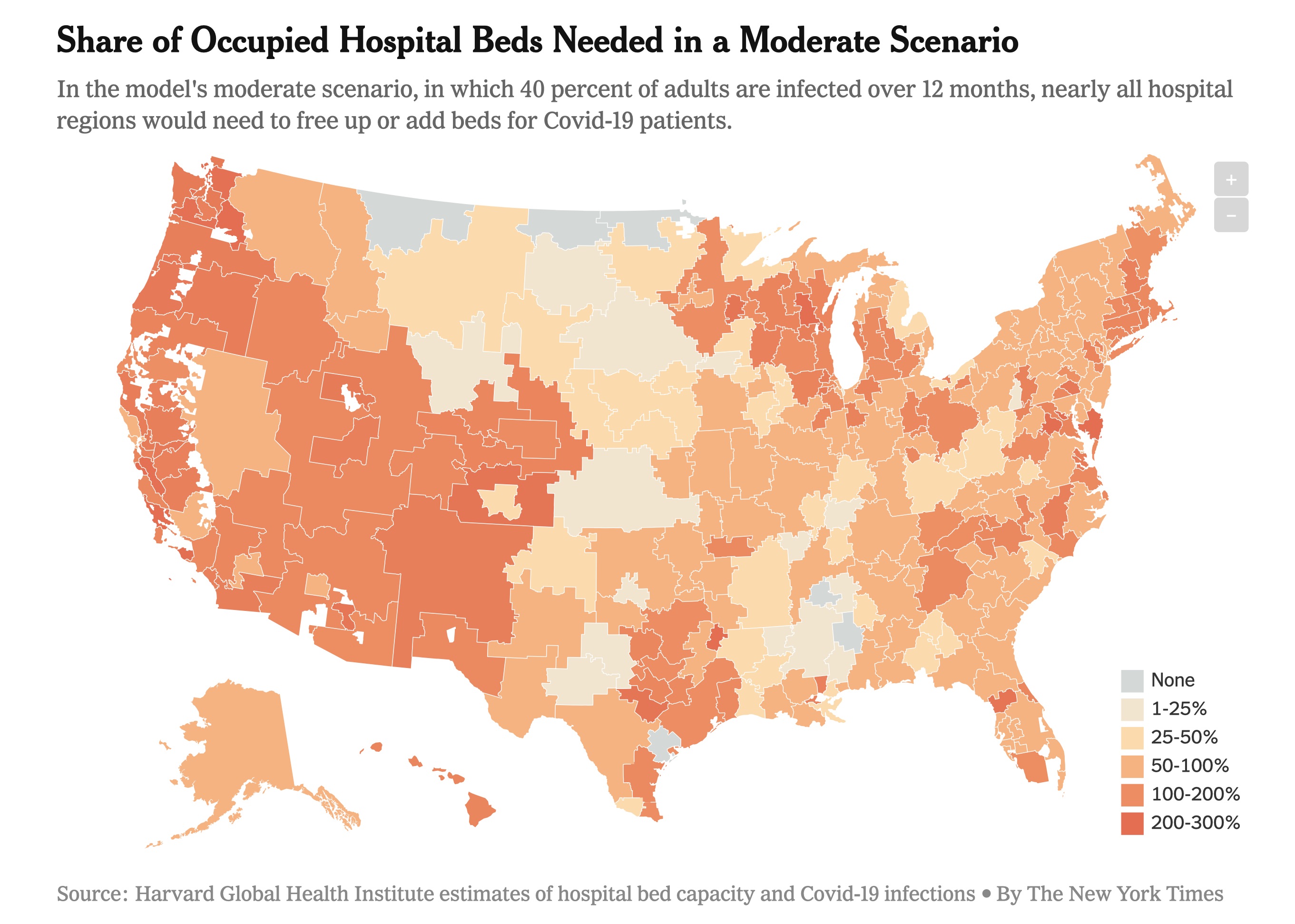 |
If 40% of adults are infected over 12 months our hospitals can barely stay under the threshold of 738,000 hospital beds in the U.S. Hopefully this is a worst-case scenario since the model does not take into account the efforts hospitals can make to quickly increase capacity during an emergency. But based on reports of what is happening to Italy’s health care system, this is what we are trying to avoid.
News & Views from my sofa:
A study published March 19, 2020 provided an updated estimate of the fatality rate of COVID-19 in Wuhan, China as of February 29, 2020. 1.4% (95% confidence interval 0.9%-2.1%) of symptomatic cases died. This is less than initial estimates. However, because of the exponential spread we are seeing outside of China, 1.4% of millions of cases is a lot of deaths.
Johns Hopkins University is hosting a map of confirmed cases, deaths, and recoveries.
An epidemiologist’s blog post explains the reproduction number, R0 or “R naught”, which describes the transmissibility of SARS-CoV-2. I’ve written about R0 previously on the March 16, 2020 update.
Simulations show that undocumented infection facilitates the spread of SARS-CoV-2. By using documented cases, mobility data, and statistical models one study showed 86% (95% confidence interval of 82%-90%) of viral infections were undocumented before China’s January 23 travel restrictions. Undocumented cases are likely not severely symptomatic and therefore shed fewer viral particles. Even though these undocumented cases have a lower transmission rate than that of very sick documented cases, the sheer number of undocumented cases means they are the source of 79% of documented cases. This is more evidence that the identification and isolation of undocumented infections will be necessary to control the outbreak. Also more reason to stay home if at all possible.
I received a question about Gilead and Regeneron’s drug development work that is attracting a lot of investment and media attention. Gilead is in charge of Phase 3 studies for the antiviral remdesivir which I described in the March 20, 2020 Update below. Regeneron Pharmaceuticals and Sanofi have started a clinical program to evaluate a drug call Kevzara. This drug was originally intended to treat patients with rheumatoid arthritis. The drug’s mechanism of action (e.g. the way it works) is by inhibiting the interleukin-6 (IL-6) pathway. This pathway is involved with the inflammatory response of our immune systems. Here is a scientific review if you want to know more, but this science is outside of my area of expertise. Data from China suggests the IL-6 pathway is an important part of the overactive inflammatory response in the lungs of COVID-19 patients. Regeneron also announced a multi-antibody cocktail that may be ready for trials in the summer. More information here. A cautionary note, lots of things have to be considered with therapeutics before we can begin to use them at scale. This includes immediate toxicity, long term effects, and actual efficacy in terms of disarming the virus. Long timelines may seem disheartening, but they mean we won’t rush something to the clinic that does more harm than good.
University of Washington Medicine is sharing resources from their experience with COVID-19 that may be of help to other health care systems beginning to see cases.
Michigan Medicine held a town hall on March 20, 2020. A few points for my local readers.
- The hospital is ramping down non-essential clinic care and procedures. They’ve also already increased virtual care capabilities with an increase in video visits by 300% and phone visits by 150%.
- In my opinion, predictions for hospital bed occupancy look worrisome by early April if a severe outbreak continues (aka if we don’t continue to flatten the curve).
- Currently not everyone with COVID symptoms can be tested. There are limitations because of availability of testing swabs/reagents and the capacity of the testing laboratory. Therefore, prioritization of testing is required. High priority groups are patients with severe illness, immunocompromised, or persons who could infect many other high risk patients such as residents of long term care facilities. Michigan Medicine is launching same-day, in-house testing.
March 20, 2020 Update
I want to start with a little explainer on coronaviruses. It’s plural because multiple types exist! We’ve known about them since the 1960s. Coronaviruses usually cause infections in birds and mammals, and were responsible for the severe acute respiratory syndrome (SARS) outbreak in 2003 and the Middle-East respiratory syndrome (MERS) in 2012. The virus SARS-CoV-2 is responsible for the current pandemic, and is a new, or novel, coronavirus. It is called coronavirus because there are crown-like spikes on the virus surface, and corona means crown in Latin (and Spanish, hence the Corona beer logo). These spikes are critical for the virus’ entry into a host cell. Here is info about naming conventions of viruses and their associated diseases. Here is a scientific review article from 2016 on coronaviruses.
An important correction to March 19’s update which I’ve made in the text below. Some evidence shows the O blood group is at a lower risk, not higher risk of COVID-19.
Here are the latest news & views from my home office:
The editorial team at Nature Research has curated relevant scientific articles. The journal Science has curated research, commentary, and news.
The Global Initiative on Sharing All Influenza Data (GISAID) has an incredible tool for visualizing the evolution and spread of the virus from 410 publicly available viral genome sequences. Just like genetic variation happens in people, genetic variation happens in viruses due to mutations. The mutations aren’t usually enough to make the virus survive or infect differently, but it helps us create a phylogenetic tree showing how the viruses are related. The closer they are on the tree, the fewer genetic differences are between them.
This research group’s ‘situation report’ from March 13, 2020 showed the multiple independent introductions of SARS-CoV-2 in the U.S. from travel from China. The clusters of cases in Washington and California that are closely related mean those initial introductions spread in the U.S. via human to human contact. Note, this report is over a week old. They have a nice page for FAQs and Misconceptions too. I want to reiterate there is strong scientific evidence that this virus is of natural origin. The spread of conspiracy theories regarding the virus being purposely manipulated to suit the agenda of a foreign country, political party, or the media is counterproductive and honestly puts lives at risk because people take scientists’ warnings less seriously.
Dr. Francis Collins, the Director of the National Institutes of Health, our nation’s largest biomedical research funder, reiterated the importance of social distancing in his blog today. He always blogs on Tuesday and Thursday about awesome tax-payer supported research, and his blog will have some COVID-19 focus these days.
There is ongoing debate regarding the use of ibuprofen to treat COVID-19 symptoms. National Public Radio (NPR) has a nice review here. A news article from the well-respected British Medical Journal (BMJ) summarizes some of the debate against ibuprofen. As of March 19 the World Health Organization (WHO) does not recommend against the use of ibuprofen. As of now, no solid scientific evidence exists to indicate we should not use ibuprofen to treat COVID-19 symptoms.
STAT is a media company focused on journalism of the life sciences and medicine. I’ve been subscribed to their daily newsletter for years now, and I think they’re a reliable source. They’ve created a guide of the leading efforts for drug and vaccine development. Things in Phase 3 trials are closer to approval for widespread use in humans. The vaccines are all in preclinical stages, which means their approval is likely 12-18 months away.
In terms of therapeutics, I’ve seen buzz about an antiviral called remdesivir. Antivirals fight viral infections by disrupting the virus life cycle so they do not continue to replicate in a host. But antivirals don’t outright kill the viral particles like some antibiotics kill bacteria. A single case study from January (published March 5, 2020) described use of remdesivir and the patient recovered. The authors state, “Although a decision to administer remdesivir for compassionate use was based on the case patient’s worsening clinical status, randomized controlled trials are needed to determine the safety and efficacy of remdesivir and any other investigational agents for treatment of patients with 2019-nCoV infection.” Early work out of China suggested remdesivir and an anti-malarial drug called chloroquine work well in cell-lines (in vitro), but had not been tested in a living organism (in vivo). Over a week ago there were some mixed results about remdesivir, largely due to this pre-print. Remdesvir is now officially in clinical trials, so time will tell. In terms of other drugs, a randomized controlled trial (the gold standard for understanding therapeutic benefit of a treatment) failed for combination therapy of two antivirals in China. You can read the New England Journal of Medicine paper or the NY Times coverage.
Call to Action: The EU Commissioner Thierry Breton is calling on streaming services like Netflix to switch to standard definition to ease the burden on the internet with many persons working from home and video chatting with co-workers, family, and friends. You as a consumer can do your part by switching to standard definition yourself when you start streaming a video. More here.
March 19, 2020 Update
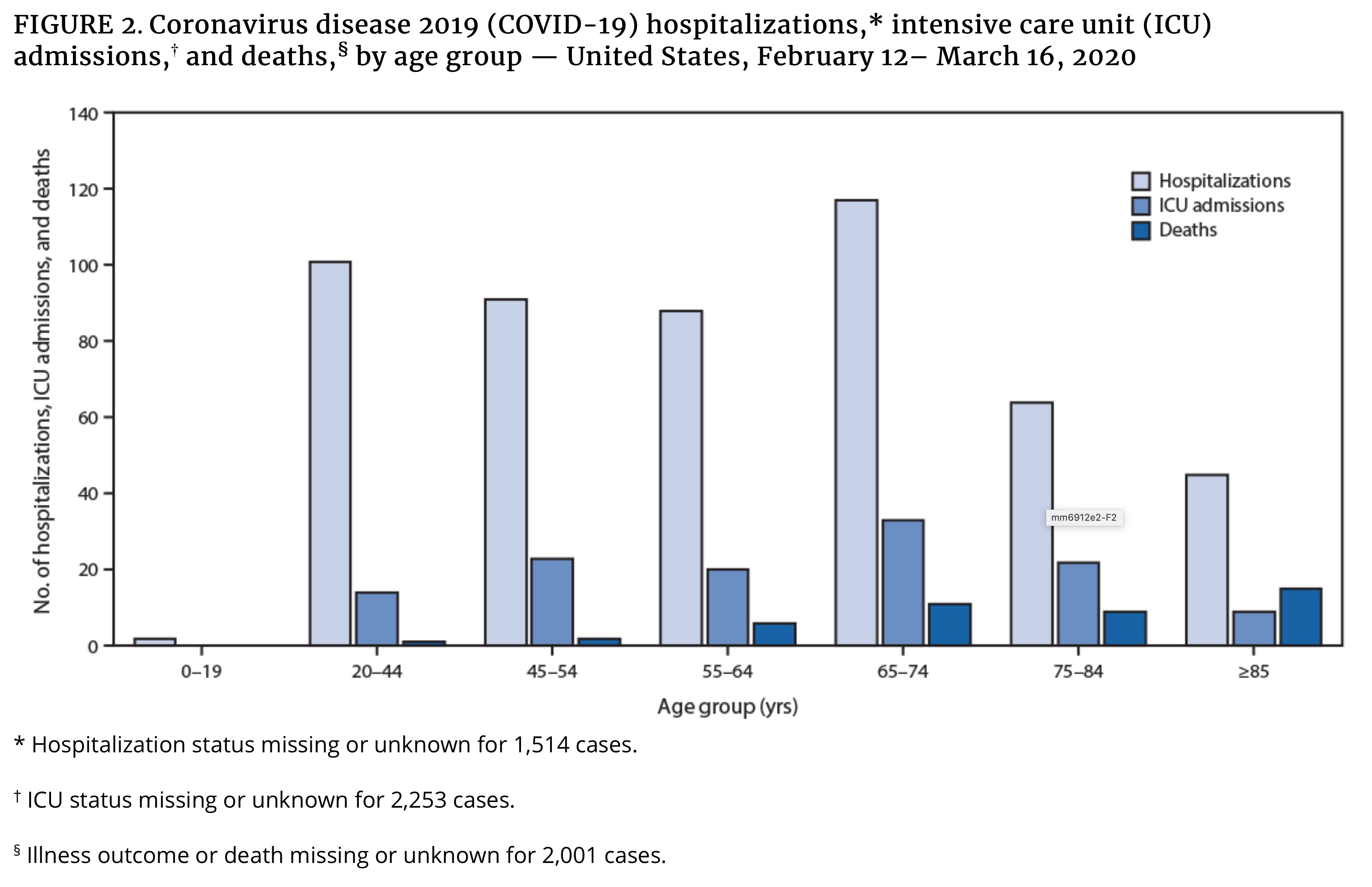
- CDC released their Mortality and Morbidity Weekly Report on March 18 showing an initial look at U.S. COVID-19 outcomes. Highlights:
- “These preliminary data also demonstrate that severe illness leading to hospitalization, including ICU admission and death, can occur in adults of any age with COVID-19.”
- 20% of hospitalizations were of persons aged 20-44 years, proving the young are not as invincible as they might believe. This is where I’d like to reiterate to milliennials and Gen Z-ers still continuing to have unnecessary social contacts: we’re all in this together, so act like it.
 |
A scientist from Seattle has a Twitter thread outlining an approach to combat the U.S. epidemic that may shorten the 18 month time frame now being reported. It starts with a huge roll out of testing to stop the spread that is occurring before symptoms begin. This would be supported with contact tracing by cell-phone location. Serological assays could be used to identify who has recovered from COVID-19 and therefore are likely to possess ongoing immunity. Those individuals with confirmed immunity could the keep economies, companies, and hospitals running. Of the many innovative ideas people are considering, it will be interesting to see what policies actually make it to implementation.
Scientific pre-prints are bringing valuable information to a global force of scientists, policy makers, and informed citizens. A quick description of pre-prints, these publications pass initial screenings by pre-print servers (e.g. medrxiv, biorxiv) but are not peer-reviewed by scientists, an important step to publishing in a respected academic research journal. In other words, they should not be trusted explicitly, but often hold important information. A recent pre-print by Jiao Zhao et al showed that individuals of the A blood group may be at higher risk of COVID-19 when compared to the AB, B, and O groups combined. The odds ratio is 1.21 with a 95% confidence interval of (1.02,1.43). This would mean the odds of COVID-19 are 21% greater in the A blood group compared to the reference group, which in this case is all other blood types. Due to a small-ish sample size of 1,775, some statisticians might say this estimate has a rather large confidence interval. This means we are 95% sure the truth is acually that the odds are anywhere from 2% to 43% greater for A blood group individuals. Note, odds≠probabilty. Conversely, individuals of the O blood group may be at lower risk when compared to A, AB, and B groups combined. The odds ratio is 0.67 with a 95% confidence interval of 0.60-0.75. This means the odds of COVID-19 are 33% less in the O blood group compared to those of other groups. More studies are needed to confirm this in other populations and in a larger sample size, but it could inform clinical guidelines or prioritizaiton of personal protective equipment (PPE) amidst scarcity for health care workers. We are all still at risk of COVID, so this should not change individual behaviors regardless of blood type.
A Chinese charitable organization, Jack Ma Foundation, and the philanthrophic arm of the Alibaba Group released a handbook of COVID-19 Prevention and Treatment aimed at health care workers.
The director of the National Institute of Mental Health released a statement on coping with coronavirus. Here is a collection of mental health resources I’ve seen shared:
